
Напишите уравнения реакций следующих превращений:
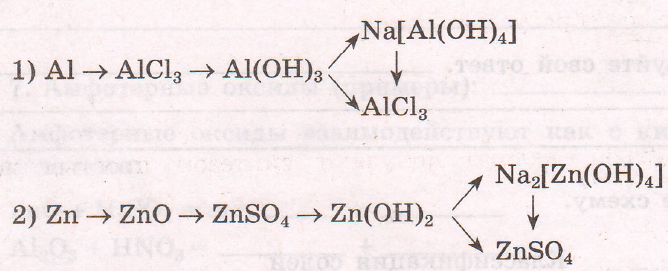

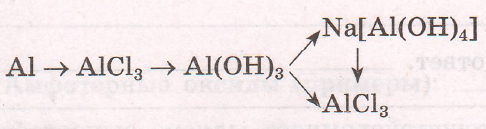
$2Al + 3Cl_{2} = 2AlCl_{3}$
$AlCl_{3} + 3NaOH = 3NaCl + Al(OH)_{3}$
$Al(OH)_{3} + NaOH = Na[Al(OH)_{4}]$
$Al(OH)_{3} + 3HCl = AlCl_{3} + 3H_{2}O$
$Na[Al(OH)_{4} + 4HCl = NaCl + AlCl_{3} + 4H_{2}O$
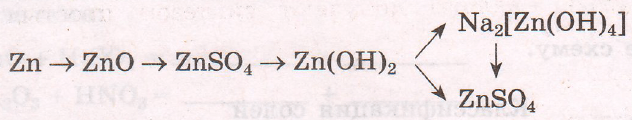
$2Zn + O_{2} = 2ZnO$
$ZnO + H_{2}SO_{4} = ZnSO_{4} + H_{2}O$
$ZnSO_{4} + 2NaOH = Na_{2}SO_{4} + Zn(OH)_{2}$
$Zn(OH)_{2} + 2NaOH = Na_{2}[Zn(OH)_{4}]$
$Zn(OH)_{2} + H_{2}SO_{4} = ZnSO_{4} + 2H_{2}O$
$Na_{2}[Zn(OH)_{4}] + 3H_{2}SO_{4} = 2NaHSO_{4} + ZnSO_{4} + 4H_{2}O$